Renata PLC, a publicly traded listed drug maker, has got approval from the European authorities to market a tablet — Cabergoline 0.5 mg — used for the treatment of hyperprolactinemia and Parkinson's disease.
The Bangladeshi leading pharmaceutical company announced the approval from the EU through the decentralised procedure (DCP) on Monday in a filing with the Dhaka Stock Exchange (DSE).
This approval comes with access to several European markets, such as Ireland, France, Portugal, Italy, Denmark, Sweden, the Netherlands, Norway and Spain.
Cabergoline, a dopamine agonist, is primarily used in the treatment of conditions such as hyperprolactinemia and Parkinson's disease, the company said.
Renata said in the official disclosure, that cabergoline 0.5 mg will be manufactured at Renata's state-of-the-art UK MHRA-approved potent facility located at Rajendrapur in Gazipur, which adheres to stringent quality control measures and is equipped to meet the demands of the European market.
This product will be distributed across Europe through various strategic partnerships, ensuring widespread patient access, it said.
Furthermore, this product, qualified for global markets, is available in the local market of Bangladesh under the name Cabolin, ensuring that patients have access to the same high-quality treatment options across the board, the company noted.
Earlier, Renata extended its footprint in the United States when it availed of the regulatory approval to enter the sophisticated US drug market.
The US Food and Drug Administration (FDA) recently approved Renata’s Gazipur-based General Plant-2 facility, a recognition that allows the drug maker to export medicines to the US market.
According to the DSE filing, the FDA recently approved Renata’s plant after a long process of inspections. This approval allowed the local drug maker to export Metoprolol Tartrate tablets to the US market.
The latest approval from the EU will also enable the company to strengthen its footprint in the global markets.
Renata, which currently exports medicines to more than 30 countries, started its operations as Pfizer (Bangladesh) Limited in 1972. However, in 1993, Pfizer transferred ownership of its operations to local shareholders, and the company name was changed to Renata.
The drug manufacturer was enlisted on the stock exchanges in 1979. Its stock price increased by 0.16 per cent to Tk 770.20 at Dhaka bourse on Monday.
Renata is one of the fastest-growing pharmaceutical and animal health product manufacturers in Bangladesh. It is also a contract manufacturer for UNICEF and SMC for general products, such as birth control pills, oral saline and micronutrient powders.
The company has 10 manufacturing facilities inside its three sites at Mirpur in Dhaka, Rajendrapur in Gazipur, and Bhaluka in Mymensingh.
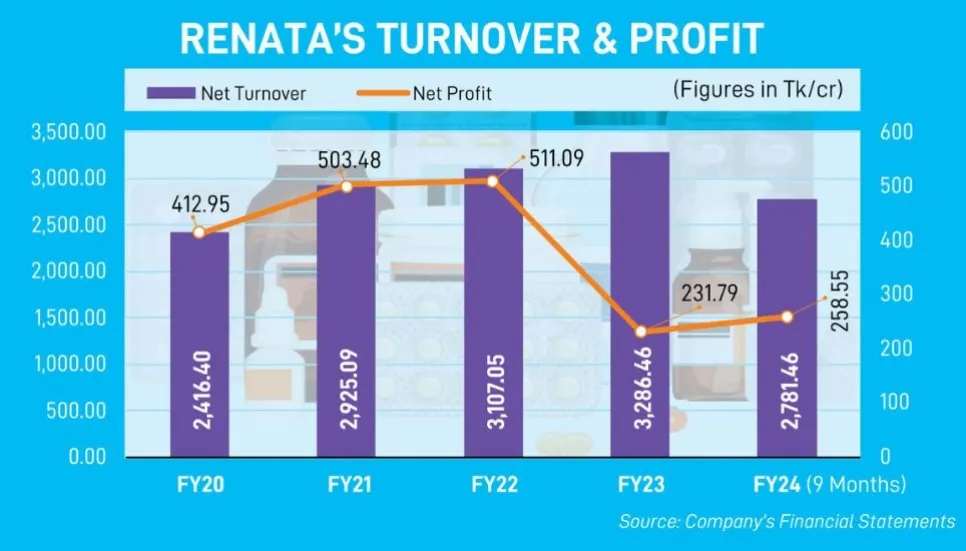
The leading drug manufacturer witnessed a 54.8 per cent year-on-year drop in net profit in FY2022-23, even though its revenue grew by 6 per cent in the year.
The situation, according to the company, was credited to the increase in the prices of raw materials and the rise in energy prices caused by the global dollar crisis.
The publicly traded company declared a 62.5 per cent cash dividend for FY23 to its shareholders, the lowest in the last seven years.
In the January-March quarter of FY2023-24, Renata reported a 21.4 per cent growth in profit and its revenue grew by 9 per cent against the rates recorded during the same period of FY23.
During Q3, it logged Tk 72.62 crore in profit and Tk 921.844 crore in revenue, according to its consolidated interim financial statements for the nine months of FY24.